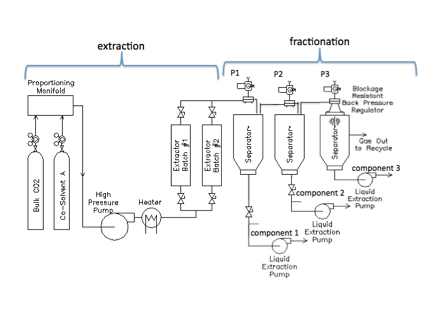
In chemistry, fractionation is one of the processes by which a mixture can be separated into its individual components.
In the case of extracting essential oils from plants, researchers have found that unique healing or medicinal properties exist in different oils within a single plant. Fractionation is used to extract each oil separately. One way to do this is by using supercritical Carbon Dioxide (sCO2). Since the plant’s component oils have different dissolution conditions in sCO2, adjusting the process pressure following supercritical extraction allows each substance to separate independently.
Supercritical Carbon Dioxide is especially useful in fractionation due to its ability to change solvent power with small pressure and temperature changes. In addition, sCO2 has the benefit of possessing environmentally friendly attributes. It is also abundant, reusable and non-toxic, and it reaches a supercritical point at an easily controlled temperature and pressure. Once at the supercritical point, the solvating properties can be adjusted with relatively small changes in temperature and pressure.
Supercritical Extraction and Fractional Separation
The process works in the following way: SCO2 is pumped through a heater to reach the pressure and temperature conditions required for solvency of the plant oils. Supercritical extraction takes place as the sCO2 moves through the plant matter in the batch extractor. In the process of fractionation, the mixture goes through a series of separator vessels in a multistage operation allowing selective and sequential fluid extraction. Each of the separators is set at a very specific pressure, allowing only one component of the mixture to drop out.
The unique capability of the Equilibar back pressure regulator to maintain precise pressure control makes it ideal for this targeted fractionation process. Moreover, Equilibar’s supercritical blockage resistant regulator is particularly useful in the final stage of the separation, where the sCO2 is decompressed for recycling. During this phase, the expansion of the supercritical fluid causes a dramatic temperature drop that can lead to ice formation and other blockages. The blockage resistant regulator features a patent pending technology to resist the build up of ice in its internal passages. The design also resists blockage from oils that have become highly viscous due to the extreme cold of the expanding gasses.
For a more detailed explanation of fractionation, see Supercritical Extraction and Fractional Separation.